Competency #3: Vaccine Development and Evaluation
This competency integrates into practice knowledge about the main steps in vaccine development and evaluation.
Learning Objectives
The health professional will be able to perform the following:
- Describe, in general terms, the process to obtain marketing approval for vaccines in Canada.
- Describe what can be learned about vaccines after they are approved for marketing, via surveillance activities and more formal post-marketing studies.
- Characterize, in broad terms, the key roles and responsibilities for each of the following relative to the postmarketing assessment of vaccine safety and effectiveness:
- Vaccine manufacturers
- Canadian regulatory authority (Biologics and Genetic Therapies Directorate)
- Public Health Agency of Canada
- Provincial/territorial Health departments
- Vaccine providers
- Healthcare providers who don’t administer vaccines
- Vaccine recipients or their parents/caregivers
Vaccine Development
Vaccine Safety
Vaccine safety is of the highest importance and concern for all vaccine stakeholders. Knowledge of vaccine development, clinical trials, and the surveillance system will help health care providers communicate the safety of vaccines which in turn will build public confidence in vaccinations.
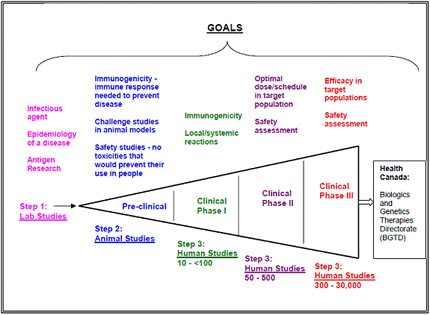
- Vaccines must be thoroughly tested before they can be called safe and effective for human use.
- It can take up to 10 years to test and develop a vaccine.
- Table 1describes the stages of vaccine development from the lab to Health Canada approval.
Table 1 Stages of Vaccine Development
Canadian Vaccine Licensing
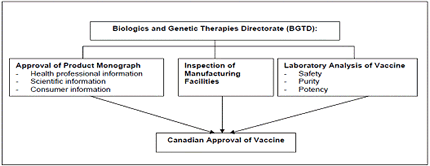
When the pharmaceutical company has successfully conducted the lab studies, animal studies, and human studies, the vaccine must meet Canadian licensing standards before the vaccine can be considered for use in Canada.
The Biologics and Genetic Therapies Directorate (BGTD) under Health Canada is the Canadian authority that regulates biological drugs (products derived from living sources) for human use.
The BGTD…
- inspect and regulate manufacturing plants.
- review and assess the data provided by the manufacturer from clinical trials
- verify that the product meets standards of safety and efficacy
- Approve the vaccine & the product monograph submitted by the manufacturer
- Conducts pre-release testing of every lot of vaccine
- Monitors safety after release with the Public Health Agency of Canada (PHAC) & the manufacturers
Table 2 Canadian Vaccine Licensing
Source for this section: BCCDC Immunization Competency Program
Other Resources for Learning
- Vaccine Safety – Canadian Immunization Guide
- Public Health Agency of Canada – Vaccine Safety
- Public Health Agency of Canada – Canadian Adverse Events Following Immunization Surveillance System (CAEFISS)
- Vaccine Safety – Canadian Pediatric Society
- Canada’s eight-component vaccine safety system: A primer for health care workers
- How the flu shot makes its way from the laboratory to the doctor’s office. Health Canada
